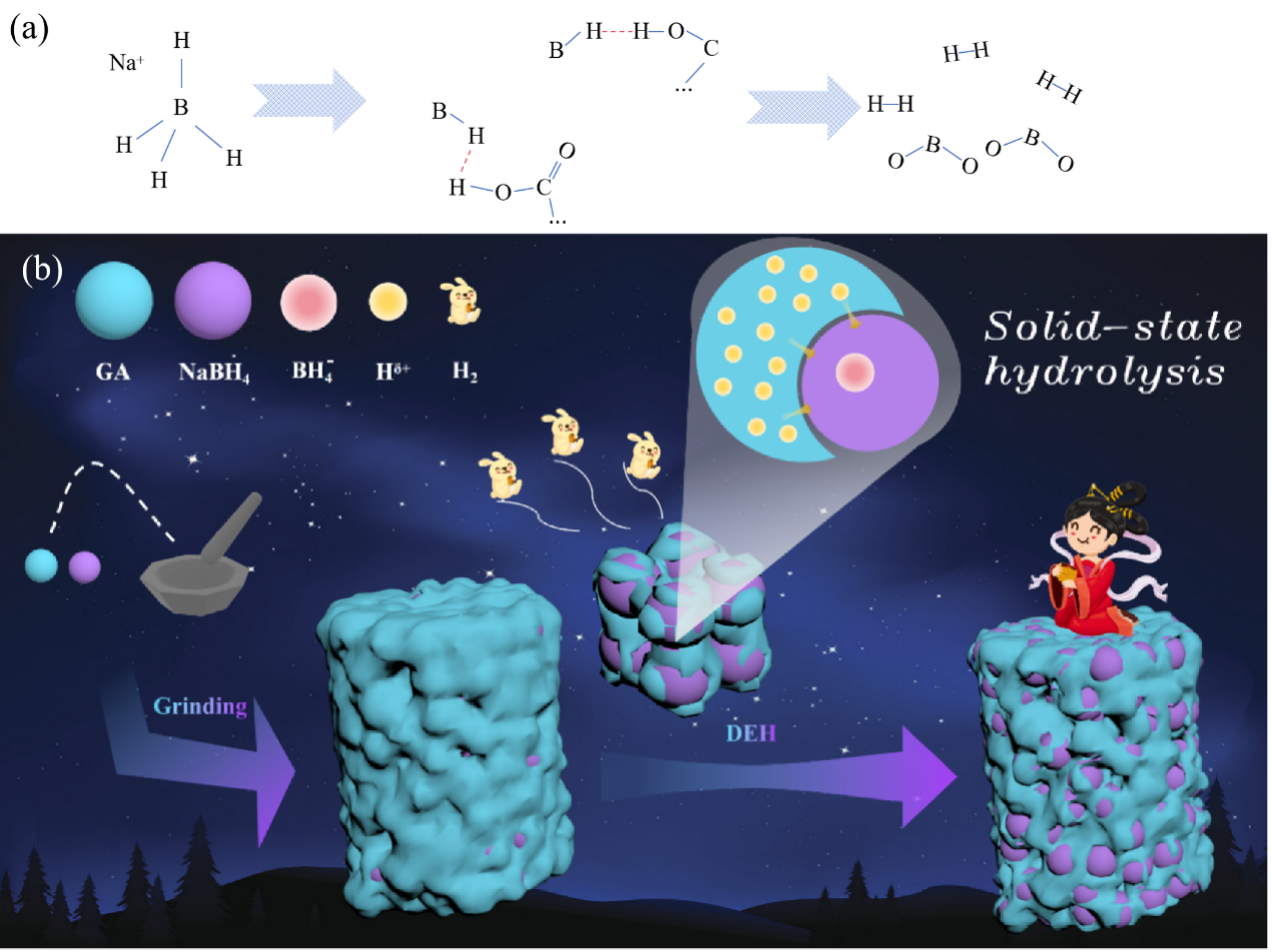
近期,我院教师本科生汤德钰(第一作者)、郑家广(通讯作者)等的研究成果“Achieving solid-state room temperature dehydrogenation from sodium borohydride composited with glycolic acid”在《Journal of Alloys and Compounds》(IF=5.8)上发表。
论文简介如下:
硼氢化钠(NaBH4)长期以来一直被认为是一种经济的储氢材料,理论储氢容量为10.6 wt%。然而,其实际应用受到热力学稳定性的阻碍,传统的NaBH4制氢需要过量的水,这降低了其体积和质量容量。在这项研究中,首次使用固态乙醇酸(GA)来失稳NaBH4以生产氢气。结果表明,NaBH4-GA从室温开始迅速释放氢气,在100℃以下产生超过3.6 wt%的氢气,氢气的有效利用率为95%。此外,NaBH4-GA复合材料在整个脱氢过程中保持固态,体积容量超过39 gH2 L-1。通过对NaBH4-GA复合材料键合结构和组成的演变的研究,进一步证实脱氢过程与NaBH4的水解过程相似,但没有出现液体。这种化学过程“固态水解”可能为使用NaBH4进行低温固态储氢提供潜在的应用和新的见解。
Sodium borohydride (NaBH4) has long been regarded as a cost-effective hydrogen storage material with a theoretical hydrogen storage capacity of 10.6 wt%. However, its practical application is hindered by thermodynamic stability, and traditional hydrogen production from NaBH4 required excess water, which lowered its volumetric and gravimetric capacities. In this study, for the first time, solid-state glycolic acid (GA) was used to destabilize NaBH4 for producing hydrogen. According to the results, NaBH4-GA started releasing hydrogen rapidly from room temperature and more than 3.6 wt% hydrogen was produced below 100 ℃ with efficient hydrogen utilization rate of 95%. Moreover, NaBH4-GA composites maintained solid-state through the entire dehydrogenation process with high volumetric capacity above 39 g H2 L-1. Through the evolution of bonding structures and composition in NaBH4-GA composites, the dehydrogenation process was further confirmed as similar to the hydrolysis process of NaBH4, but with no liquid appearing. This chemical process “solid-state hydrolysis” may offer potential application and novel insights in low-temperature solid-state hydrogen storage using NaBH4.