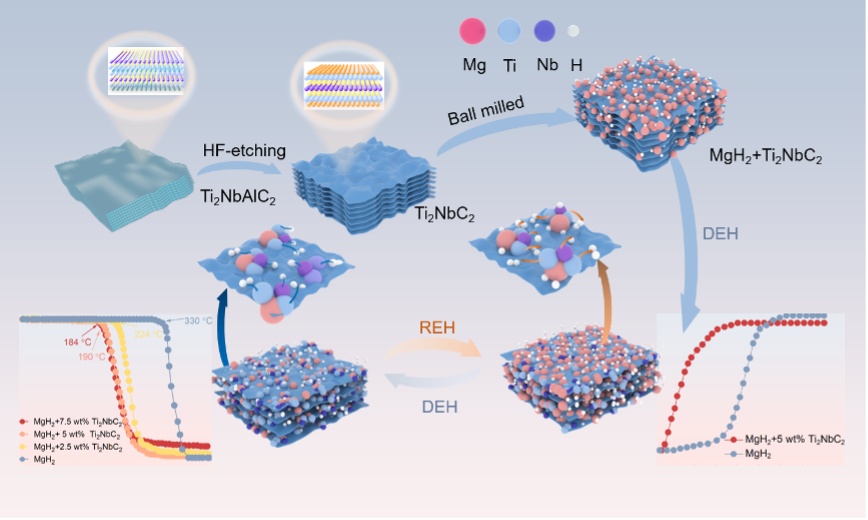
论文简介如下:
氢化镁(MgH2) 因其高达 7.6 wt% 的大量储氢能力而受到高度评价,但其商业应用受到高工作温度和缓慢动力学的阻碍。在本研究中,层状 Ti2NbC2的成功合成显著提高了 MgH2的储氢性能。MgH2 + 5 wt% Ti2NbC2在 190 °C 开始释放氢气,并在室温下开始吸收氢气。在 275 °C 的恒定温度下,仅需 250 秒即可完全释放氢气,最高可达 6.9 wt%。在 150 °C 时,氢气的吸收在 200 s 内达到 6.59 wt%,氢气吸收活化能降低到 41.517 ± 3.981 kJ·mol−1,动力学性能显著提高。此外,复合材料在 275 °C 下循环 20 次后仍表现出优异的循环稳定性。在 MgH2对 Ti2NbC2进行氢脱氢/吸收的过程中,活性物质 Nb-H 和 Ti-H 在原位生成,有效地削弱了 Mg-H 键,并充当高效的“氢泵”,加速 MgH2的再/脱氢。Ti2NbC2独特的层状结构和氢亲和力为氢迁移提供了有效的转移通道,这是 MgH2 + Ti2NbC2优异储氢性能的关键。
Magnesium hydride (MgH2) was highly regarded for its substantial hydrogen storage capacity of up to 7.6 wt%, but its commercial application was hindered by the high operating temperatures and slow kinetics. In this study, the successful synthesis of the layered Ti2NbC2 has significantly enhanced the hydrogen storage performance of MgH2. MgH2 + 5 wt% Ti2NbC2 began to release hydrogen at 190 °C and started to absorb hydrogen at room temperature. At a constant temperature of 275 °C, complete hydrogen release was achieved in just 250 s, up to 6.9 wt%. At 150 °C, the absorption of hydrogen reached 6.59 wt% within 200 s, and the hydrogen absorption activation energy was reduced to 41.517 ± 3.981 kJ·mol−1, significantly improving the kinetic performance. Moreover, the composite material still exhibited excellent cyclic stability after 20 cycles at 275 °C. In the process of hydrogen de/absorption of Ti2NbC2 with MgH2, active substances Nb-H and Ti-H were generated in situ, which effectively weakened the Mg-H bond and acted as efficient “hydrogen pumps” to accelerate the re/dehydrogenation of MgH2. The unique layered structure and hydrogen affinity of Ti2NbC2provided an effective transfer channel for hydrogen migration, which was key to the excellent hydrogen storage performance of the MgH2 + Ti2NbC2.