近期,我院研究生马镇璇(第一作者)、教师郑家广(通讯作者)等的研究成果“Enhanced hydrogen storage performance of Mg(BH4)2with in-situ generated TiO2 and TiH2 catalysts”在中科院一区期刊《Chemical Engineering Journal》(IF=13.3)上发表。
论文简介如下:
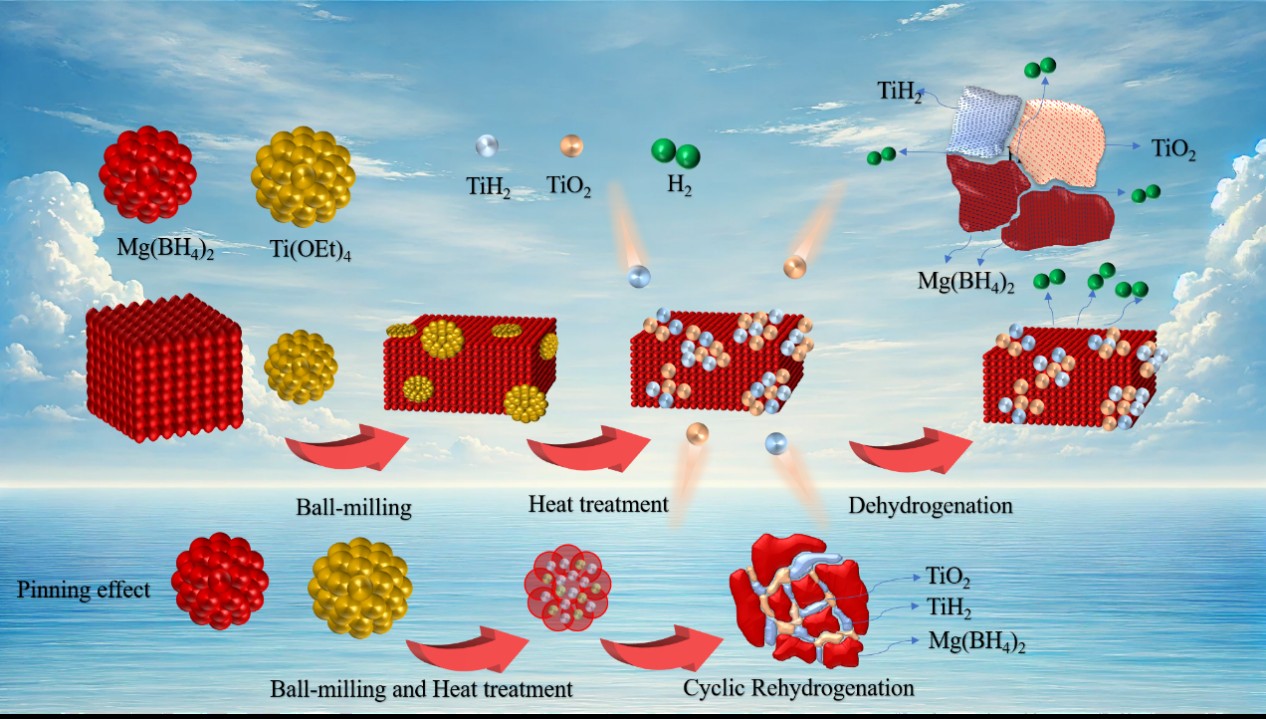
硼氢化镁是一种极具发展前景的固态储氢材料,由于其脱氢动力学缓慢,可逆性差,其实际应用仍有待进一步研究。本工作,选择钛酸四乙酯(TET)作为液态催化前驱体,并通过机械球磨和热处理工艺构将其掺入Mg(BH4)2中原位形成的TiO2和TiH2催化剂。与未掺杂Mg(BH4)2相比,Mg(BH4)2-10% TET复合材料的初始脱氢温度降低了100 ℃以上。等温脱氢曲线和动力学拟合计算表明Mg(BH4)2-10%TET复合材料的两步氢释放活化能降至252.58 kJ∙mol-1和144.15 kJ∙mol-1。循环脱氢试验表明,经过4个循环后与未掺杂Mg(BH4)2相比,Mg(BH4)2- 10% TET样品的可逆性提高了75%。脱氢过程中,掺杂TET后原位形成的TiH2和TiO2均匀分布在Mg(BH4)2颗粒上。TiH2可以作为“氢泵”加速氢的快速释放,TiO2也可以作为催化剂实现有效的电子转移。在Mg(BH4)2-10%TET复合过程中,形成了大量的相边界和界面,有利于氢沿这些界面的扩散和移动。这些研究为提高硼氢化镁的储氢性能提供了一条有效途径。
Magnesium borohydride was a highly promising solid-state hydrogen storage material, but its actual use still faced challenge due to its sluggish dehydrogenation kinetics and poor reversibility. In this study, the Tetraethyl titanate (TET) was chosen as a liquid-state catalytic precursor and was incorporated into Mg(BH4)2 through ball milling and thermal treatment process to build in-situ formed TiO2 and TiH2 dopants. The initial dehydrogenation temperature of Mg(BH4)2-10%TET composite was reduced by more than 100 °C comparing to that of un-doped Mg(BH4)2. The isothermal dehydrogenation curves and kinetic fitting calculations indicated that the activation energy for the two-step hydrogen release of Mg(BH4)2-10%TET composite was reduced to 252.58 kJ∙mol-1 and 144.15 kJ∙mol-1, respectively. Cyclic dehydrogenation tests demonstrated that after four cycles, the reversibility of Mg(BH4)2-10%TET sample was improved by 75% compared to the un-doped Mg(BH4)2. During the dehydrogenation process, in-situ formed TiH2 and TiO2 after doping TET were uniformly distributed on Mg(BH4)2 particles. TiH2 could act as a “hydrogen pump” to accelerate the rapid release of hydrogen, and TiO2 could also achieve effective electron transfer as a catalyst.Moreover, during the Mg(BH4)2-10%TET composite, a significant number of phase boundaries and interfaces were formed, which facilitated the diffusion and movement of hydrogen along these interfaces. These studies exhibited an effective approach for improving the hydrogen storage performance of magnesium borohydride.